FDA 21 CFR Part 11 Training, Regulations, and Best Practices - GxP Training : Certified Online Courses for Life Sciences
![Attachment 1. Excerpts from Federal Food and Drug Administration regulations [Code of Federal Regulations] [Title 21, Volume 2] Attachment 1. Excerpts from Federal Food and Drug Administration regulations [Code of Federal Regulations] [Title 21, Volume 2]](https://forloveofwater.org/wp-content/uploads/2017/07/Attachment-1-1-pdf.jpg)
Attachment 1. Excerpts from Federal Food and Drug Administration regulations [Code of Federal Regulations] [Title 21, Volume 2]



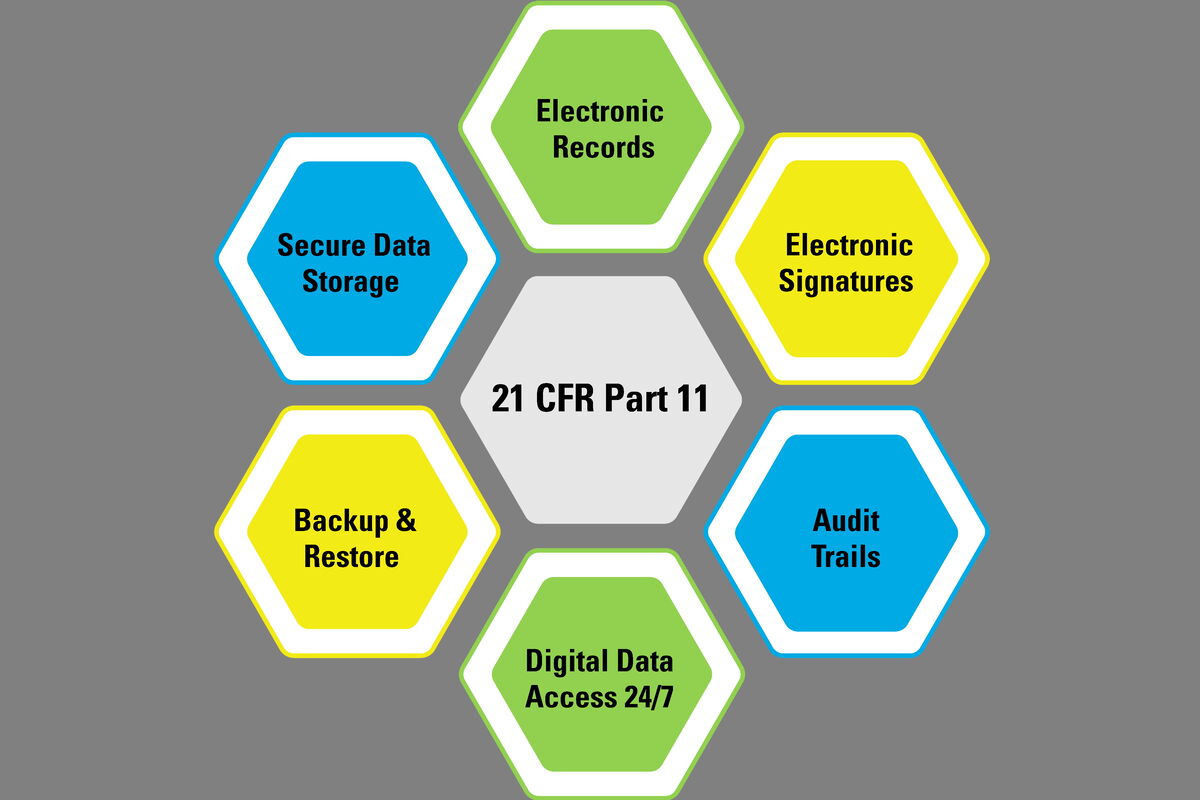
















%20Complete%20Guide%20to%2021%20CFR%20Part%2011.png?width=1700&name=(cover)%20Complete%20Guide%20to%2021%20CFR%20Part%2011.png)